Abstract
Background:
Venetoclax (ven)-based time-limited combination treatments have yielded high rates of undetectable MRD (uMRD) in patients (pts) with CLL. In correlative analyses, attainment of uMRD status was associated with longer progression-free survival (PFS), making uMRD a robust surrogate parameter for remission duration particularly after time-limited therapy. While MRD is usually assessed by conventional 4-color flow cytometry (FCM) defining uMRD as less than 1 CLL cell in 10 000 leukocytes (<10 -4, uMRD4), a better prognostic discrimination by more sensitive methods appears feasible. In addition to conventional 4-color FCM, we assessed uMRD5 (<10 -5) and uMRD6 (<10 -6) with high-sensitivity FCM (hsFCM) and next-generation sequencing (NGS) of immunoglobulin genes in the GAIA (CLL13) trial.
Methods:
The phase 3 GAIA (CLL13) trial compared 3 different time-limited ven-based combinations against chemoimmunotherapy (CIT) in fit, treatment-naïve pts with CLL. Pts were randomized to receive CIT for 6 cycles of 28 days each (FCR for pts ≤65 years; BR for pts >65 years), ven and rituximab (RVe), ven and obinutuzumab (GVe), or ven, obinutuzumab and ibrutinib (GIVe), all for 12 cycles with the option for ibrutinib continuation until cycle 36 for pts not obtaining uMRD4. The co-primary endpoints were the rate of uMRD4 at month (MO) 15 (GVe vs CIT) and PFS (GIVe vs CIT). MRD was centrally assessed by FCM at MO2, MO9, MO12 and MO15 in peripheral blood (PB) and at final restaging (RE, two months after the end of treatment) in bone marrow (BM). The following categories were used: high (≥10 -2), intermediate (≥10 -4 to <10 -2), uMRD4 (<10 -4). Exploratory post hoc MRD analyses were performed by hsFCM and/or an amplicon-based one-step NGS protocol by the EuroClonalityNGS group. HsFCM data was generated by reevaluation of FCM data files and reducing the cut off for CLL events to at least 10 events in 2 of 3 MRD tubes. MRD data were evaluated with regard to categories of uMRD5 and uMRD6.
Results:
In total, 926 pts were randomized (CIT: 229, RVe: 237, GVe: 229, GIVe: 231). Based on the intention-to-treat (ITT) population, rates of uMRD4 in PB by FCM were 62.0% (CIT), 73.0% (RVe), 88.6% (GVe) and 88.3% (GIVe) at MO9 and 52.0% (CIT), 57.0 (RVe), 86.5% (GVe) and 92.2% (GIVe) at MO15. BM uMRD4 results at RE were 37.1% (CIT), 43.0 (RVe), 72.5% (GVe) and 77.9% (GIVe).
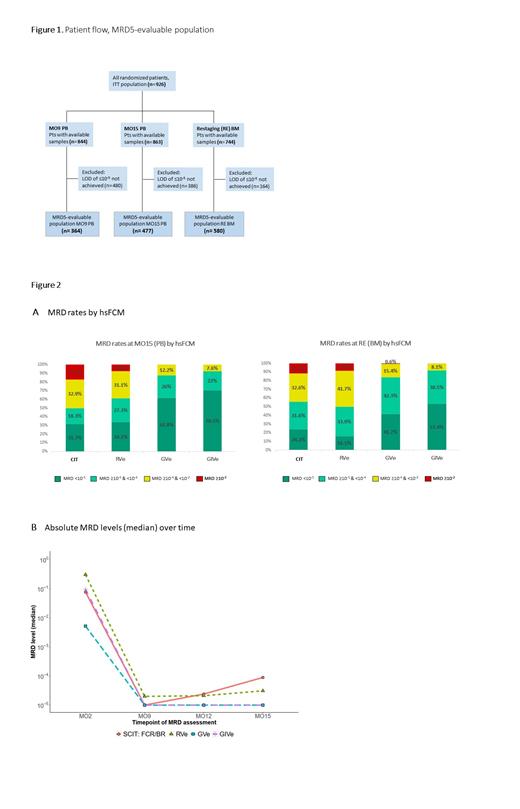
HsFCM samples were available for 844 (MO9 PB), 863 (MO15 PB) and 744 (RE BM) pts with median limits of detection (LOD) of 1.6x10 -5 (MO9 PB), 1.4x10 -5 (MO15 PB) and 9.9x10 -6 (RE BM) that were similar between the treatment arms. With hsFCM a lower limit of detection (LOD) of ≤10 -5 was achieved in 364 (MO9) and 477 (MO15) PB samples and in 580 BM samples at RE. 480 (MO9 PB), 386 (MO15 PB) and 164 (RE BM) samples did not reach a LOD of ≤10 -5 and were thus not included in the MRD5-evaluable populations (Figure 1).
Among pts with samples evaluable for MRD5 in PB at MO15, 26 of 82 (31.7%, CIT), 45 of 132 (34.1%, RVe), 81 of 131 (61.8%, GVe) and 93 of 132 (70.5%, GIVe) achieved uMRD5. BM uMRD5 rates at RE were 24.2% (23 of 95 pts), 16.1% (27 of 168 pts), 41.7% (65 of 156 pts) and 53.4% (86 of 161 pts), respectively (Figure 2A).
The median MRD level at MO9 was lower in CIT, GVe, GIVe (all 1x 10 -5) compared with RVe (2x 10 -5) by hsFCM (Figure 2B). While the obinutuzumab-containing arms stayed at this low level between MO9 and MO15, median MRD levels in CIT and RVe increased to 8.9x 10 -5 (CIT) and 3.1x 10 -5 (RVe) in the same period.
The different treatment arms showed distinct patterns of differential clearance of CLL in BM and PB. While the fraction of concordant MRD results between PB and BM at RE was lower in the CIT arm with 14/34 (41.2%), the ven-containing arms showed a similar compartment effect with a proportion of concordant results of 67/108 (62.0%, RVe), 70/101 (69.3%, GVe) and 71/104 (68.3%, GIVe). In 9/16 (56.3%, CIT), 23/36 (63.9%, RVe), 22/63 (34.9%, GVe) and 23/73 (31.5%,GIVe) pts who achieved uMRD5 in PB (RE) MRD was still measurable in BM.
More sensitive NGS analyses and detailed correlative analyses are pending and will be presented at the conference.
Conclusions:
HsFCM improves MRD detection in CLL below 10 -4 in PB and BM by capturing low levels of MRD (≥10 -5 to <10 -4) in samples that were assessed as uMRD4 by FCM. HsFCM was able to show differences in uMRD rates between the treatment arms more clearly than FCM. Obinutuzumab-containing arms and in particular the GIVe arm showed the highest uMRD5 rates in PB and BM.
Von Tresckow: Celgene: Other: travel grant; AstraZeneca: Honoraria, Other; Roche: Honoraria, Other: Reasearch support, travel grant; Janssen: Honoraria, Other: Reasearch support, travel grant; AbbVie: Honoraria, Other: advisory board, travel grant. Niemann: CSL Behring, Genmab, Takeda, Octapharma: Consultancy; Abbvie, AstraZeneca, Janssen: Consultancy, Research Funding; Novo Nordisk Foundation: Research Funding. Kater: Genmab, LAVA: Other: Ad Board, Steering Committee; Abbvie: Honoraria, Other: Ad Board, Research Funding; BMS, Roche/Genentech: Other: Ad Board, , Research Funding; Janssen, AstraZeneca: Other: Ad Board, steering committee, Research Funding. Simon: Gilead: Other: Travel support. Fink: AbbVie: Other: travel grant; Janssen: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Celgene: Research Funding. Fischer: Abbvie: Honoraria; Roche: Honoraria, Other: Travel Grants. Wendtner: Mundipharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; F. Hoffmann-LaRoche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding. Staber: Roche: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Takeda: Consultancy, Research Funding; MSD: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria. Tadmor: Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Levin: Roche, Janssen, Abbvie: Other: Travel Expenses, Ad-Board. Stilgenbauer: AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, Sunesis: Honoraria; AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, Sunesis: Other: Research Support; AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, Sunesis: Research Funding; AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, Sunesis: Consultancy. Brüggemann: Amgen: Other: Advisory Board, Travel support, Research Funding, Speakers Bureau; Incyte: Other: Advisory Board; Janssen: Speakers Bureau. Hallek: Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau. Ritgen: MSD: Consultancy, Other: Travel support; Chugai: Consultancy; Abbvie: Consultancy, Other: Travel support, Research Funding; Roche: Consultancy, Other: Travel support, Research Funding; Celgene: Other: Travel support. Eichhorst: AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodation, expenses, Research Funding, Speakers Bureau; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodation, expenses, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodation, expenses, Speakers Bureau; Adaptive Biotechnologies: Speakers Bureau; Hexal: Speakers Bureau; ArQule: Membership on an entity's Board of Directors or advisory committees; Oxford Biomedica (UK): Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodation, expenses, Research Funding, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodation, expenses, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Travel, accomodation, expenses, Research Funding, Speakers Bureau; Consultant Department I for Internal Medicine: Consultancy; University Hospital of Cologne: Current Employment.
The combination of obinutuzumab, venetoclax and ibrutinib is not approved for treatment of CLL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal